RNA Programs
Providing Biotech and Pharma Companies with Technology for Effective and Safe Delivery of RNA to Extrahepatic Tissues
Altamira’s xPhore™ technology is a versatile platform that allows complexation of various types of RNA molecules with a proprietary peptide to form nanoparticles. These nanoparticles allow delivering RNA therapeutics by systemic or local administration to diseased tissues via leaky vasculature, a hallmark of solid tumors and certain inflammatory conditions. xPhore™ technology has been extensively and successfully tested with various RNA modalities in almost 20 in vivo disease models.
We are developing our xPhore™ platform for RNA delivery with two strategic aims: 1) to support partners in biotech and pharma with the development and manufacturing of nanoparticles for delivery of their own RNA, and 2) to demonstrate the potential of xPhore™ through two proprietary development programs in the preclinical and early clinical stage (“flagship programs”).
Indications for xPhore™
| Cancer Models | Target | RNA |
|---|---|---|
Pancreatic and colorectal cancer | KRAS | siRNA |
Ovarian cancer | TAM: AXL | siRNA |
Lung cancer | ETV-2 | siRNA |
Metastatic melanoma | NF-κB | siRNA |
Adult T-cell Leukemia / Lymphoma (ATLL) | NF-κB | siRNA |
Sarcoma | MYCT-1 | siRNA |
Sarcoma and breast cancer | MYCT-1 | siRNA |
Tumor microenvironment | ZBTB46 | mRNA |
| Cardiovascular | ||
|---|---|---|
Artherosclerosis | JNK2 | siRNA |
Atherosclerosis | p27Kip1 | mRNA |
Aortic aneurysm | NF-κB | siRNA |
Aortic aneurysm | SOD2 | mRNA |
| Inflammation / Autoimmune / Metabolic | ||
|---|---|---|
Necrotizing enterocolitis | NF-κB | siRNA |
Rheumatoid and osteoarthritis | NF-κB | siRNA |
Metabolic syndrome / obesity | ASXL2 | siRNA |
Osteoarthritis | WNT16 | mRNA |
And the list keeps growing…
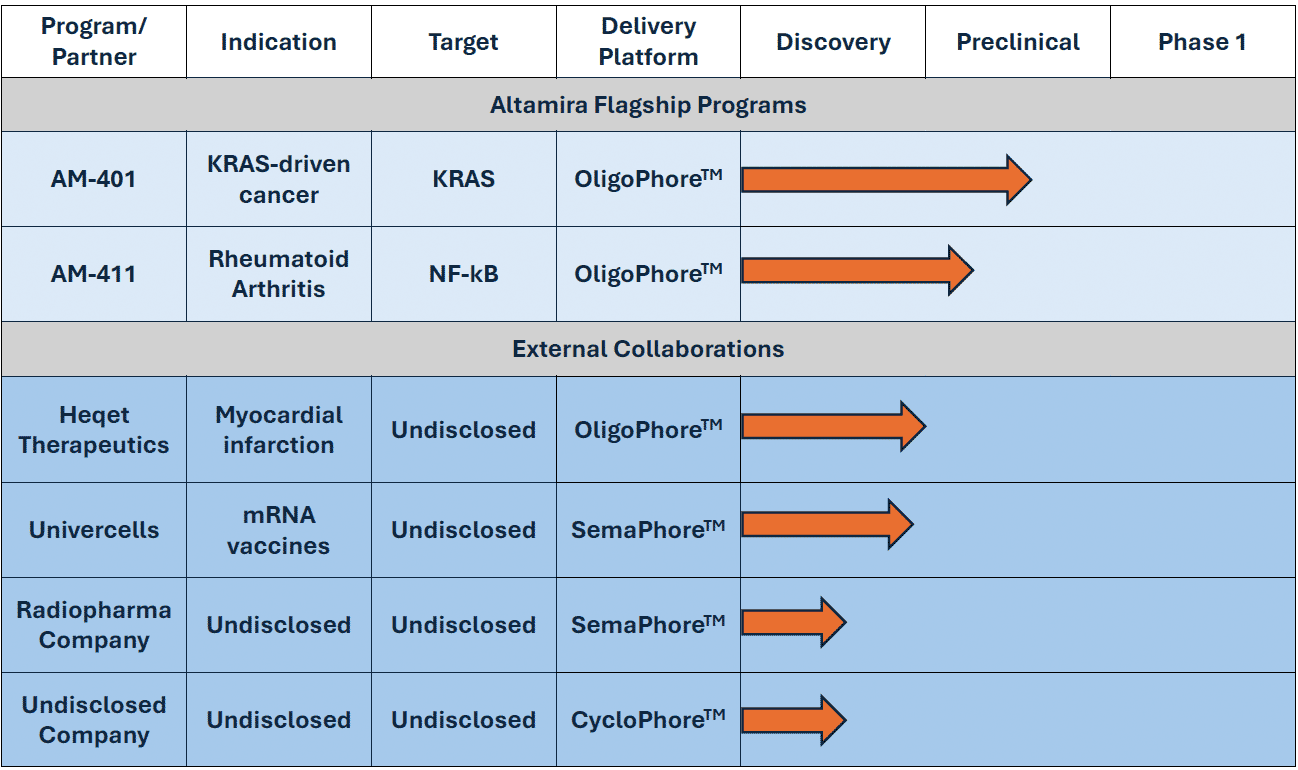
Development Pipeline
-
AM-401 for the treatment of KRAS-driven cancers
-
AM-411 for the treatment of rheumatoid arthritis
Flagship Programs
AM-401: KRAS-Driven Cancers
The KRAS gene encodes the K-Ras protein which controls - like an “on / off switch” – cell growth, maturation, migration and cell death. Through mutations, the K-Ras proteins can be rendered persistently active, causing cancer cells to proliferate and spread in the body. Mutations of KRAS are associated with poor prognosis in several cancers, and there is a substantial body of evidence supporting the role of KRAS in the initiation and maintenance of cancer.
There are more than a dozen different KRAS mutations in human cancer, but the prevalence of each mutation is variable among different types of cancers. Mutated forms of KRAS are found in one-fifth of all human cancers, including 32% of non-small-cell lung cancers, 40% of colorectal cancers and 85–90% of pancreatic cancers (Herdeis et al., 2021). According to the American Cancer Society, almost 150,000 new cases of KRAS mutated tumors are diagnosed in the United States alone across these three tumors types each year. It has been estimated that mutations in KRAS alone account for approximately one million deaths per year worldwide (Simanshu et al., 2017).
AM-401 is an intravenously administered nanoparticle based on the OligoPhore™ platform targeting KRAS. In contrast to small molecule inhibitors, that are designed to inhibit KRAS harboring one specific mutation, Altamira’s approach, polyKRASmut, is polyvalent as it allows for silencing KRAS carrying any of the most abundant mutations described for this oncogene. Proof-of-concept has been obtained in well-established mouse models of pancreatic cancer; and biodistribution studies have shown specific delivery of the siRNA to the tumor tissue. Altamira plans to file an IND in 2026.
polyKRASmut-OligoPhore nanoparticles inhibit tumor growth in a mouse model of pancreatic cancer. Tumor volume following treatment of KPC-1 tumor bearing mice starting one-week post-tumor engraftment with KRAS-OligoPhore nanoparticles or scrambled counterparts. Strand et al., 2019
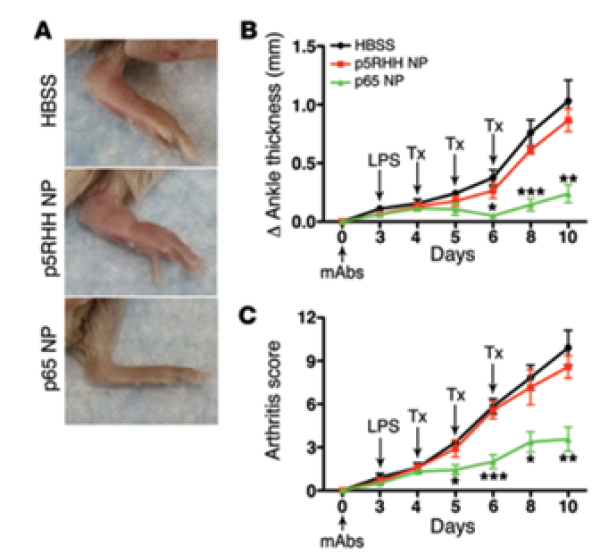
AM-411: Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory condition causing joint swelling and pain which may also affect other areas, including the skin, eyes, brain, and cardiovascular system. In the US in 2014, approximately 1.3 million adults suffered from RA (Hunter et al., 2017) and according to the World Health Organization (WHO), the autoimmune disease affects globally up to 18 million people. RA affects 1 in 28 women and 1 in 59 men during their lifetime (Crowson et al., 2012).
There is no cure for RA; current treatments seek to manage RA with biologic and non-biologic immunosuppressants, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs). While useful, drug resistance occurs in up to 50% of patients and systemic adverse reactions are frequent, including rash, hair loss, altered liver function, low blood cell counts, nausea, increased infections and neuropathy. New biologics targeting JAK/interleukins have been issued black box warnings by the FDA. The global antirheumatics market is expected to grow from $57.9 billion in 2019 to $62.9 billion in 2027 (Allied Market Research).
AM-411 is an intravenously administered nanoparticle based on the OligoPhore™ platform targeting p65, one of the components of NF-kB and a key checkpoint in RA inflammation. AM-411 has been designed to reduce local inflammation without affecting the NF-κB pathway outside the inflamed tissue and is less likely to generate resistance because it reduces synthesis of p65 rather than blocking the protein. An IND filing is planned for 2026.
Reduction of inflammation, ankle thickness and arthritis score in response to p65-OligoPhore nanoparticles compared to scrambled siRNA nanoparticles or HBSS. Zhou et al., 2014
Partnering
Altamira’s xPhore™ platform offers solutions for extrahepatic delivery of RNA therapeutics and vaccines. Advantages include protection of the therapeutic cargo from degradation and clearance, access to extrahepatic tissues and highly efficient endosomal release. Altamira is open to strategic collaborations with parties interested in:
-
In-licensing/collaboration on our xPhore™ delivery technology for specific payloads, indications, or tissues (OligoPhore™, SemaPhore™, CycloPhore™).
-
In-licensing/collaboration on our siRNA therapeutic candidates AM-401 (targeting polyKRASmut in KRAS-driven cancers) or AM-411 (targeting NF-kB in rheumatoid arthritis).
For inquiries about partnership opportunities please contact